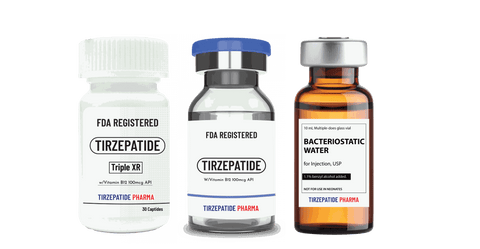
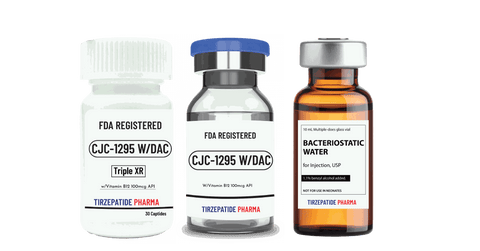
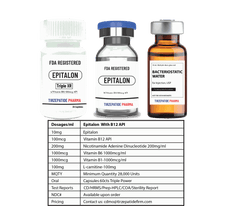
White Label minimum quantity for FDA-registered peptides with Methyl Vitamin B12 (1000mcg API)/ml is 50,000 packs for companies interested in ANDA drug application. Manufactured with approved DMF according to the Federal Food, Drug, and Cosmetic Act for commercial distribution, ANDA registration, or clinical trials.
Combining innovative peptide science and flexible GMP manufacturing capabilities. Tirzepatide Pharma offers GMP-compliant synthesis services for peptide NCEs and commercial peptides. We offer GMP-compliant synthesis services for peptide NCEs and commercial peptides. We provide optimized and scaled-up drug substance. Our peptide development and manufacturing platform provides comprehensive CRDMO/CMO services, capable of handling a diverse range of peptide projects.






 Pin it
Pin it