Clinical Commercial Peptide APIs. Our teams have developed, scaled up, and manufactured up to 200 kg of GMP peptide APIs using the solid-phase process, as well as up to 50 kg of peptide APIs through convergent solid and solution-phase synthesis processes. We also develop and manufacture custom peptides, New Drug Peptide Entities, Encapsulated peptides, and oral lozenges under current Good Manufacturing Practice (cGMP) conditions.
White Label minimum quantity for FDA-registered peptides with Methyl Vitamin B12 (1000mcg API)/ml is 50,000 packs for companies interested in ANDA drug application. Manufactured with approved DMF according to the Federal Food, Drug, and Cosmetic Act for commercial distribution, ANDA registration, or clinical trials..






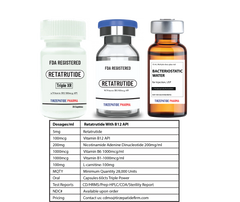
 Pin it
Pin it