Tirzepatide Pharma's peptide drug discovery service addresses key concerns, including immunogenicity, poor pharmacokinetics, lack of oral bioavailability, and high manufacturing costs. Utilizing our proprietary technologies and expertise, we develop peptide drugs that are both effective and commercially viable.
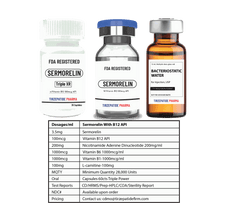
White Label minimum quantity for FDA-registered peptides with Methyl Vitamin B12 (1000mcg API)/ml is 50,000 packs for companies interested in ANDA drug application.. Manufactured with approved DMF according to the Federal Food, Drug, and Cosmetic Act for commercial distribution, ANDA registration, or clinical trials.




 Pin it
Pin it