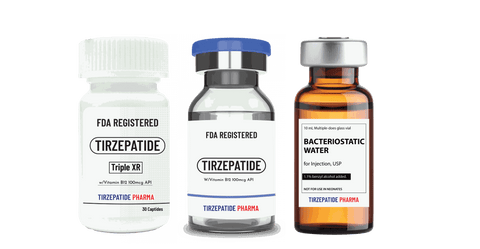
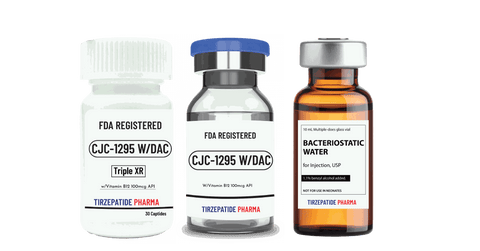
One-to-One Dedicated Team. No matter where your project starts, Tirzepatide Pharma is the partner you need for a fully integrated solution, from preclinical to commercial stages. We eliminate the hassle of managing multiple providers by offering:
- One point of contact
- One clear path to project completion
- One partner for API & Drug Product development
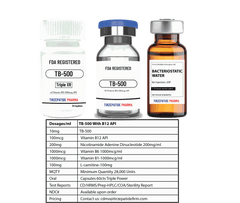
White Label minimum quantity for FDA-registered peptides with Methyl Vitamin B12 (1000mcg API)/ml is 50,000 packs for companies interested in ANDA drug application. Manufactured with approved DMF according to the Federal Food, Drug, and Cosmetic Act for commercial distribution, ANDA registration, or clinical trials.





 Pin it
Pin it